Kiomedine® CM-Chitosan is a highly purified polysaccharide derived from Agaricus Bisporus (button mushroom) resulting from years of research and innovation and is a patented technologysup>1,2>/sup> manufactured by KiOmed Pharma in Belgium.
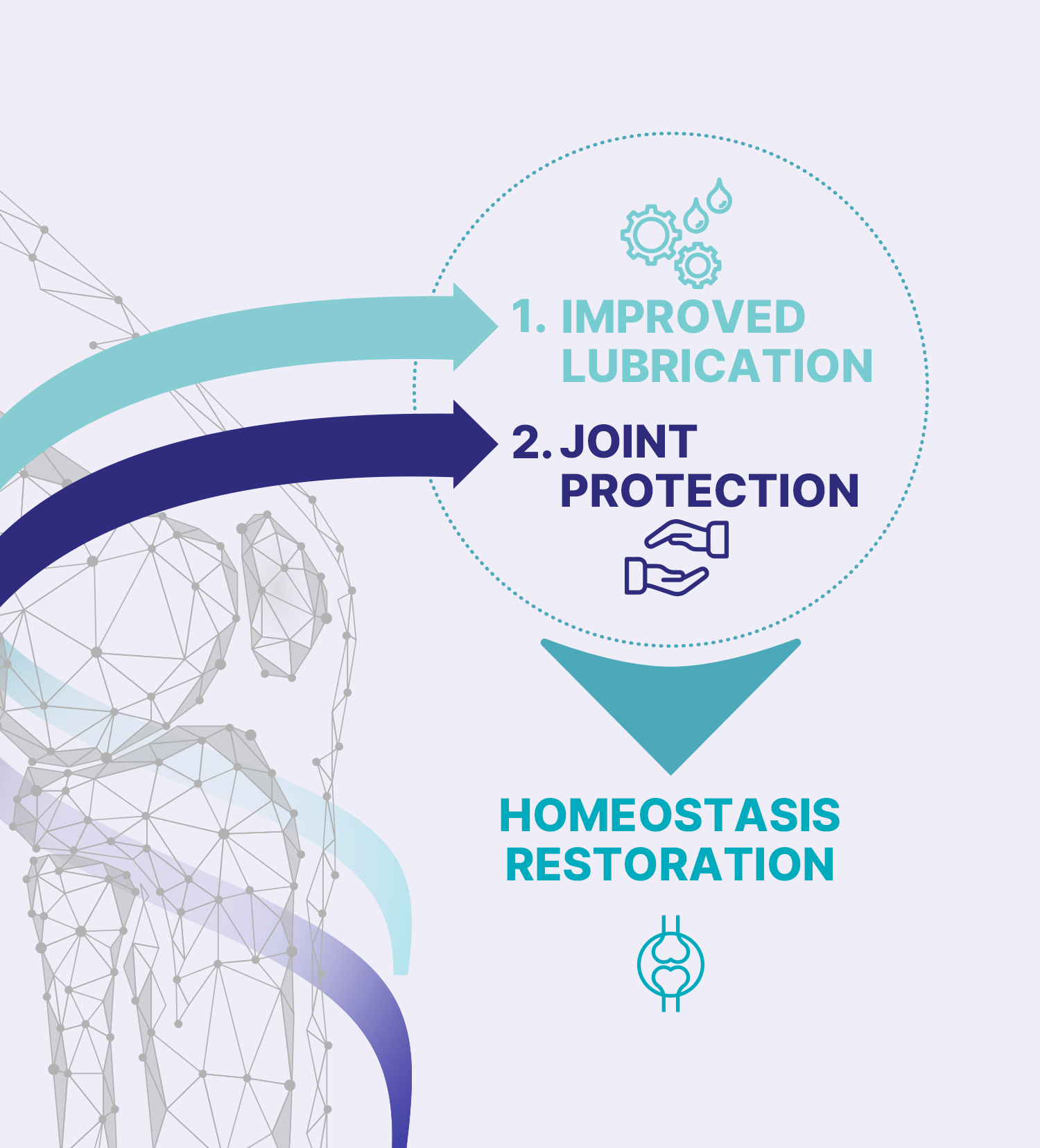
Thanks to a unique structure that differs from natural chitosan, CM-Chitosan provides to KioMedineVSone an exclusive dual mechanism of action to tackle osteoarthritis pain and other symptoms by enhancing joint lubrication and by reducing oxidative stress (protection)3,4.
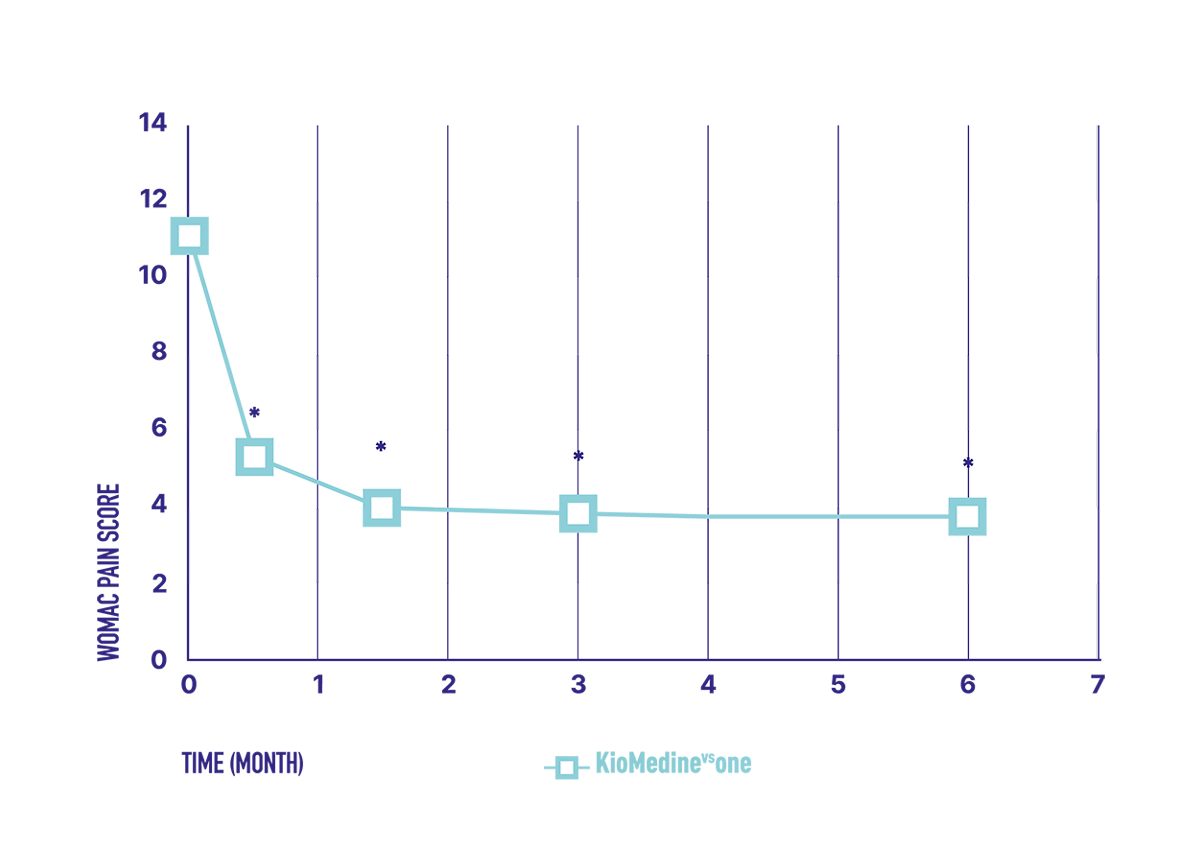
The lubrication and ROS scavenger properties of KioMedinevsone are properties potentially helping to disrupt the OA vicious cycle, and therefore providing relief of the OA-related symptoms on the long-term.